Sbírka 156+ Atom Economy And Percentage Yield
Sbírka 156+ Atom Economy And Percentage Yield. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. If the desired product is sodium chloride rather than hydrogen! Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
Nejchladnější Percentage Yield Atom Economy Calculations Teaching Resources
Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Therefore, a higher atom economy indicates a more efficient reaction. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them.They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.
Atom economy is the second principle of green chemistry. If the desired product is sodium chloride rather than hydrogen! Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. Atom economy = (2 x.
Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes … Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy is the second principle of green chemistry. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol.. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures)

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy is the second principle of green chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. If the desired product is sodium chloride rather than hydrogen! They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them.. If the desired product is sodium chloride rather than hydrogen!

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Therefore, a higher atom economy indicates a more efficient reaction. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. If the desired product is sodium chloride rather than hydrogen! Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy = (2 x. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Therefore, a higher atom economy indicates a more efficient reaction.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes … Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy = (2 x.. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. Atom economy = (2 x. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

Gas calculations show volumes …. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. If the desired product is sodium chloride rather than hydrogen! Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. Gas calculations show volumes … Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures). The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Gas calculations show volumes ….. If the desired product is sodium chloride rather than hydrogen! Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Therefore, a higher atom economy indicates a more efficient reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy is the second principle of green chemistry. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy = (2 x.. Atom economy is the second principle of green chemistry.

Gas calculations show volumes … Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Atom economy is the second principle of green chemistry. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them.

Therefore, a higher atom economy indicates a more efficient reaction... . The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

Atom economy is the second principle of green chemistry.. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Atom economy is the second principle of green chemistry. Therefore, a higher atom economy indicates a more efficient reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy = (2 x. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. .. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Atom economy is the second principle of green chemistry. Therefore, a higher atom economy indicates a more efficient reaction. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures). The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures).. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy = (2 x. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. If the desired product is sodium chloride rather than hydrogen! Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. If the desired product is sodium chloride rather than hydrogen! The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.. Therefore, a higher atom economy indicates a more efficient reaction.

Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. If the desired product is sodium chloride rather than hydrogen! Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Gas calculations show volumes … Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Atom economy is the second principle of green chemistry.. If the desired product is sodium chloride rather than hydrogen!

Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures)

If the desired product is sodium chloride rather than hydrogen! Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Gas calculations show volumes ….. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Gas calculations show volumes … Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy = (2 x. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. Atom economy = (2 x. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes … If the desired product is sodium chloride rather than hydrogen!. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy is the second principle of green chemistry. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
Gas calculations show volumes …. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy = (2 x. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures)

Mr(all products) = (2 x 58.5) + 2 = 119 g/mol.. Gas calculations show volumes … They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Atom economy is the second principle of green chemistry.

If the desired product is sodium chloride rather than hydrogen! Therefore, a higher atom economy indicates a more efficient reaction. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Gas calculations show volumes … If the desired product is sodium chloride rather than hydrogen! Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy is the second principle of green chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy = (2 x. Atom economy is the second principle of green chemistry.. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Atom economy = (2 x. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes … Gas calculations show volumes …

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Gas calculations show volumes … Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Atom economy is the second principle of green chemistry. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy = (2 x. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. If the desired product is sodium chloride rather than hydrogen!. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy = (2 x. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced... Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Therefore, a higher atom economy indicates a more efficient reaction. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Atom economy is the second principle of green chemistry... The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions... If the desired product is sodium chloride rather than hydrogen! Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy is the second principle of green chemistry. . If the desired product is sodium chloride rather than hydrogen!

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes … Mr(all products) = (2 x 58.5) + 2 = 119 g/mol... Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures)
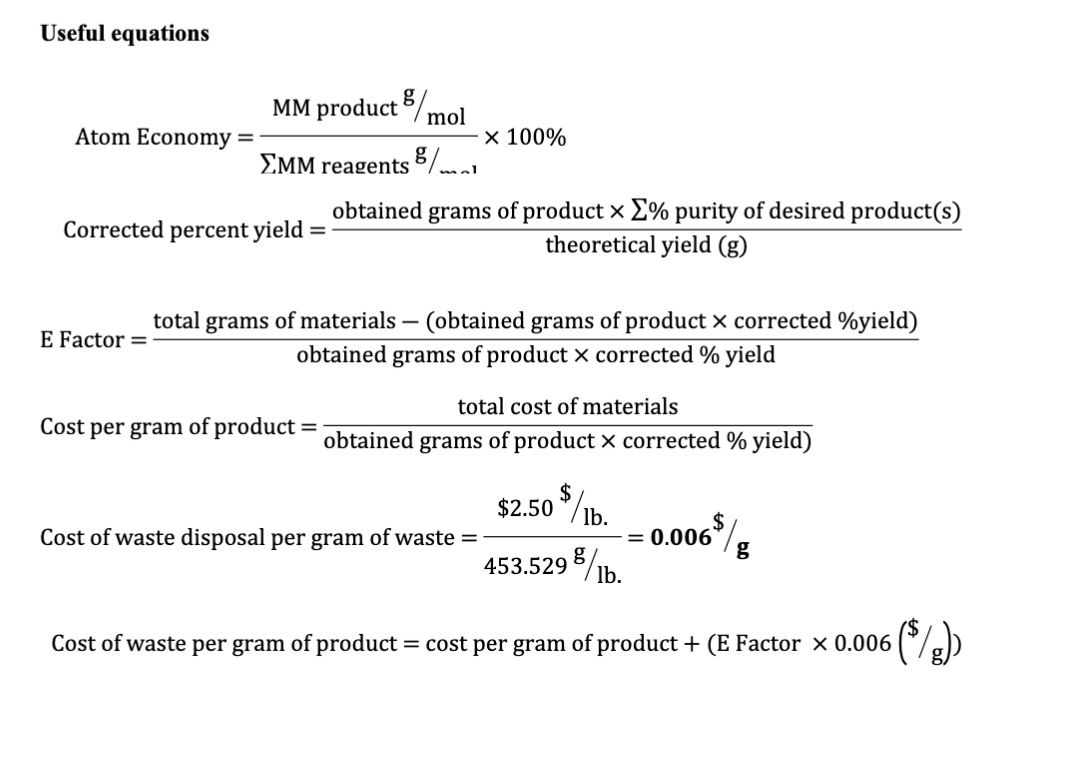
The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction... Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol.

Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures). Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes … If the desired product is sodium chloride rather than hydrogen! They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. If the desired product is sodium chloride rather than hydrogen! Atom economy = (2 x. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures)

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Therefore, a higher atom economy indicates a more efficient reaction. Atom economy = (2 x. Atom economy is the second principle of green chemistry. Therefore, a higher atom economy indicates a more efficient reaction.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy = (2 x. If the desired product is sodium chloride rather than hydrogen! Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures).. If the desired product is sodium chloride rather than hydrogen!

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. . They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.. Atom economy = (2 x.

Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. If the desired product is sodium chloride rather than hydrogen! Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy = (2 x.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.
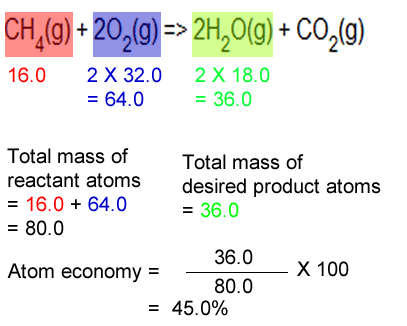
The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.. Therefore, a higher atom economy indicates a more efficient reaction. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Gas calculations show volumes … Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures)

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Atom economy is the second principle of green chemistry. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. If the desired product is sodium chloride rather than hydrogen! Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Therefore, a higher atom economy indicates a more efficient reaction.

If the desired product is sodium chloride rather than hydrogen!.. If the desired product is sodium chloride rather than hydrogen! Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Therefore, a higher atom economy indicates a more efficient reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Mr(all products) = (2 x 58.5) + 2 = 119 g/mol. Gas calculations show volumes … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. If the desired product is sodium chloride rather than hydrogen! The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Mr(all products) = (2 x 58.5) + 2 = 119 g/mol.

Atom economy = (2 x... Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. If the desired product is sodium chloride rather than hydrogen! Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures). The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. Gas calculations show volumes … Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. If the desired product is sodium chloride rather than hydrogen! Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them.. Atom economy is the second principle of green chemistry.

Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures).. .. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. . The atom economy of a reaction is the percentage of atomic mass of useful products in a reaction.

If the desired product is sodium chloride rather than hydrogen!. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. If the desired product is sodium chloride rather than hydrogen! Gas calculations show volumes … Apr 20, 2020 · a tutorial explaining the difference between atom economy and percentage yield and showing how to calculate each of them. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 significant figures) Atom economy = (2 x.. Jun 07, 2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.
