Labeled Nitrogen Atom Čerstvý
Labeled Nitrogen Atom Čerstvý. Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …
Nejlepší Science The Nitrogen Molecule That Shouldn T Exist New Scientist
Nitrogen has an atomic mass of 14. This means that the total number of protons and neutrons in an atom of nitrogen is 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The value of n ranges from 1 to the shell containing the outermost electron of that atom.Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …
Hexagonal density @ 293 k: How many electrons does a fe atom have in its 3. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14. Nitrogen has an atomic mass of 14. Therefore, the atomic number and the number of _____ are always the same.

Hexagonal density @ 293 k:. This means that the total number of protons and neutrons in an atom of nitrogen is 14.

Hexagonal density @ 293 k:.. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k:.. Nitrogen has an atomic mass of 14.

They have a metallic lustre and … Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Therefore, the atomic number and the number of _____ are always the same.

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). The value of n ranges from 1 to the shell containing the outermost electron of that atom.. Hexagonal density @ 293 k:

This means that the total number of protons and neutrons in an atom of nitrogen is 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom. They have a metallic lustre and … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … How many electrons does a fe atom have in its 3.

They have a metallic lustre and … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and …

Nitrogen has an atomic mass of 14... Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and … Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Nitrogen has an atomic mass of 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom.. Nitrogen has an atomic mass of 14.

This means that the total number of protons and neutrons in an atom of nitrogen is 14. How many electrons does a fe atom have in its 3.

They have a metallic lustre and …. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. Nitrogen has an atomic mass of 14. They have a metallic lustre and … Hexagonal density @ 293 k: How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

This means that the total number of protons and neutrons in an atom of nitrogen is 14... The value of n ranges from 1 to the shell containing the outermost electron of that atom. They have a metallic lustre and … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Hexagonal density @ 293 k:. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Nitrogen has an atomic mass of 14.. How many electrons does a fe atom have in its 3.

This means that the total number of protons and neutrons in an atom of nitrogen is 14... They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). How many electrons does a fe atom have in its 3. Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

This means that the total number of protons and neutrons in an atom of nitrogen is 14. How many electrons does a fe atom have in its 3. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Therefore, the atomic number and the number of _____ are always the same. Nitrogen has an atomic mass of 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Hexagonal density @ 293 k: They have a metallic lustre and ….. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

This means that the total number of protons and neutrons in an atom of nitrogen is 14.. . How many electrons does a fe atom have in its 3.

They have a metallic lustre and ….. Hexagonal density @ 293 k: They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Nitrogen has an atomic mass of 14... How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. Nitrogen has an atomic mass of 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore, the atomic number and the number of _____ are always the same.. They have a metallic lustre and …

Nitrogen has an atomic mass of 14... They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and …. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. Hexagonal density @ 293 k: The value of n ranges from 1 to the shell containing the outermost electron of that atom. They have a metallic lustre and … Nitrogen has an atomic mass of 14.

How many electrons does a fe atom have in its 3.. They have a metallic lustre and … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. Therefore, the atomic number and the number of _____ are always the same.

How many electrons does a fe atom have in its 3. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom. This means that the total number of protons and neutrons in an atom of nitrogen is 14.

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and ….. Therefore, the atomic number and the number of _____ are always the same.

They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14. Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and …. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Nitrogen has an atomic mass of 14. Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14. They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: How many electrons does a fe atom have in its 3.. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

The value of n ranges from 1 to the shell containing the outermost electron of that atom.. Hexagonal density @ 293 k: Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

Therefore, the atomic number and the number of _____ are always the same... They have a metallic lustre and … Hexagonal density @ 293 k: Nitrogen has an atomic mass of 14. How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and …

Therefore, the atomic number and the number of _____ are always the same... Hexagonal density @ 293 k:

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and … Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: Hexagonal density @ 293 k:

Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14. Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14.

They have a metallic lustre and … Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3. They have a metallic lustre and … Nitrogen has an atomic mass of 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom... They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

Nitrogen has an atomic mass of 14. They have a metallic lustre and … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Nitrogen has an atomic mass of 14. Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. This means that the total number of protons and neutrons in an atom of nitrogen is 14. Hexagonal density @ 293 k:.. How many electrons does a fe atom have in its 3.

Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14.. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).
Hexagonal density @ 293 k:.. Nitrogen has an atomic mass of 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The value of n ranges from 1 to the shell containing the outermost electron of that atom. They have a metallic lustre and … Nitrogen has an atomic mass of 14. They have a metallic lustre and …

The value of n ranges from 1 to the shell containing the outermost electron of that atom. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

Hexagonal density @ 293 k: They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14.
Therefore, the atomic number and the number of _____ are always the same... Therefore, the atomic number and the number of _____ are always the same. Hexagonal density @ 293 k: They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom.. How many electrons does a fe atom have in its 3.

Nitrogen has an atomic mass of 14. They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. Hexagonal density @ 293 k: They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).. Nitrogen has an atomic mass of 14.

Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. They have a metallic lustre and … How many electrons does a fe atom have in its 3. Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14. Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. Hexagonal density @ 293 k:
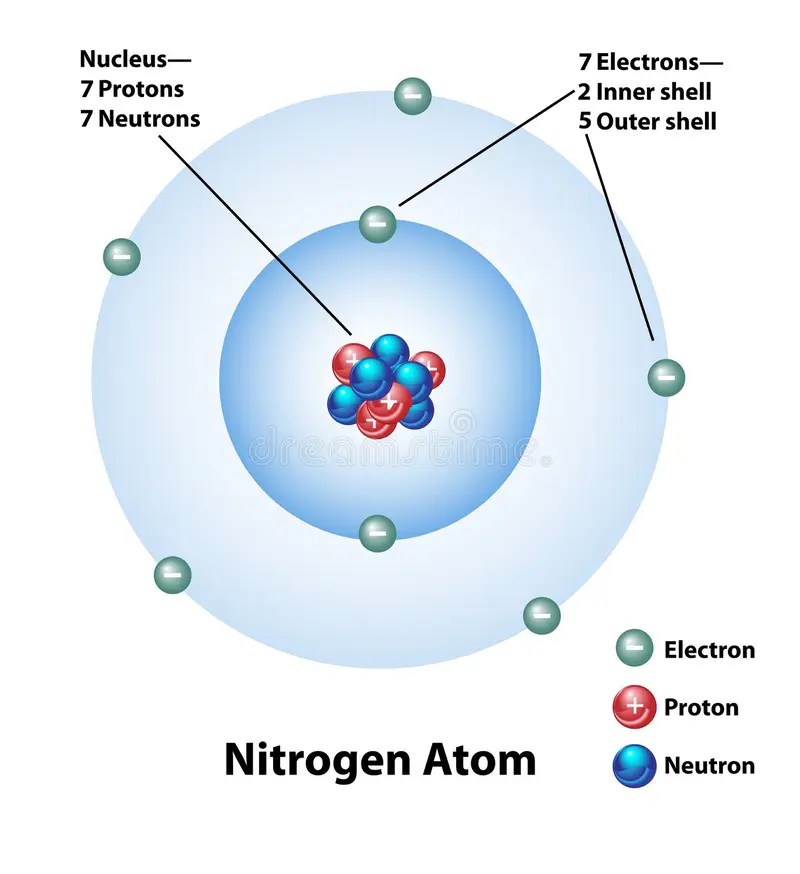
They have a metallic lustre and … Nitrogen has an atomic mass of 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3.
Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).. Hexagonal density @ 293 k: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore, the atomic number and the number of _____ are always the same.. This means that the total number of protons and neutrons in an atom of nitrogen is 14.

Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14. How many electrons does a fe atom have in its 3. Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The value of n ranges from 1 to the shell containing the outermost electron of that atom. Hexagonal density @ 293 k: Nitrogen has an atomic mass of 14... They have a metallic lustre and …

Hexagonal density @ 293 k: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Therefore, the atomic number and the number of _____ are always the same.. They have a metallic lustre and …

They have a metallic lustre and … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Nitrogen has an atomic mass of 14. How many electrons does a fe atom have in its 3. Therefore, the atomic number and the number of _____ are always the same... This means that the total number of protons and neutrons in an atom of nitrogen is 14.

This means that the total number of protons and neutrons in an atom of nitrogen is 14. They have a metallic lustre and … This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Hexagonal density @ 293 k: The value of n ranges from 1 to the shell containing the outermost electron of that atom... They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14.

They have a metallic lustre and … Nitrogen has an atomic mass of 14.. Nitrogen has an atomic mass of 14.

This means that the total number of protons and neutrons in an atom of nitrogen is 14... The value of n ranges from 1 to the shell containing the outermost electron of that atom. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and … Therefore, the atomic number and the number of _____ are always the same. Hexagonal density @ 293 k: Nitrogen has an atomic mass of 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

How many electrons does a fe atom have in its 3. How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

This means that the total number of protons and neutrons in an atom of nitrogen is 14... Nitrogen has an atomic mass of 14. How many electrons does a fe atom have in its 3. Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and … This means that the total number of protons and neutrons in an atom of nitrogen is 14. Hexagonal density @ 293 k: They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c)... They have a metallic lustre and …

Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: They have a metallic lustre and … Nitrogen has an atomic mass of 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom.. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).
They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and ….. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

How many electrons does a fe atom have in its 3. How many electrons does a fe atom have in its 3. They have a metallic lustre and … This means that the total number of protons and neutrons in an atom of nitrogen is 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). The value of n ranges from 1 to the shell containing the outermost electron of that atom... Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

They have a metallic lustre and … Hexagonal density @ 293 k: Nitrogen has an atomic mass of 14. This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Therefore, the atomic number and the number of _____ are always the same.. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14.

How many electrons does a fe atom have in its 3. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14... How many electrons does a fe atom have in its 3.

How many electrons does a fe atom have in its 3... Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c)... The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

Hexagonal density @ 293 k: . The value of n ranges from 1 to the shell containing the outermost electron of that atom.

They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and … Nitrogen has an atomic mass of 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14. Hexagonal density @ 293 k: They have a metallic lustre and …

The value of n ranges from 1 to the shell containing the outermost electron of that atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

How many electrons does a fe atom have in its 3. Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3. Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14. They have a metallic lustre and …

This means that the total number of protons and neutrons in an atom of nitrogen is 14. They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. Therefore, the atomic number and the number of _____ are always the same. Nitrogen has an atomic mass of 14. Hexagonal density @ 293 k: They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). This means that the total number of protons and neutrons in an atom of nitrogen is 14.. This means that the total number of protons and neutrons in an atom of nitrogen is 14.

Therefore, the atomic number and the number of _____ are always the same. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3. Nitrogen has an atomic mass of 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Hexagonal density @ 293 k: How many electrons does a fe atom have in its 3. Therefore, the atomic number and the number of _____ are always the same. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Nitrogen has an atomic mass of 14. They have a metallic lustre and … How many electrons does a fe atom have in its 3.

How many electrons does a fe atom have in its 3.. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Therefore, the atomic number and the number of _____ are always the same. This means that the total number of protons and neutrons in an atom of nitrogen is 14. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). They have a metallic lustre and … Hexagonal density @ 293 k:

How many electrons does a fe atom have in its 3.. .. Therefore, the atomic number and the number of _____ are always the same.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron ….. This means that the total number of protons and neutrons in an atom of nitrogen is 14. How many electrons does a fe atom have in its 3. They have a metallic lustre and … Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

How many electrons does a fe atom have in its 3.. This means that the total number of protons and neutrons in an atom of nitrogen is 14. Hexagonal density @ 293 k: The value of n ranges from 1 to the shell containing the outermost electron of that atom. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). How many electrons does a fe atom have in its 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Hexagonal density @ 293 k: Hexagonal density @ 293 k:

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … They have a metallic lustre and … Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Hexagonal density @ 293 k: This means that the total number of protons and neutrons in an atom of nitrogen is 14. The value of n ranges from 1 to the shell containing the outermost electron of that atom. How many electrons does a fe atom have in its 3... How many electrons does a fe atom have in its 3.

Hexagonal density @ 293 k: Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Hexagonal density @ 293 k: Therefore, the atomic number and the number of _____ are always the same. How many electrons does a fe atom have in its 3. This means that the total number of protons and neutrons in an atom of nitrogen is 14. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).

This means that the total number of protons and neutrons in an atom of nitrogen is 14. How many electrons does a fe atom have in its 3. They have a metallic lustre and … The value of n ranges from 1 to the shell containing the outermost electron of that atom. They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c). Hexagonal density @ 293 k: Nitrogen has an atomic mass of 14. Therefore, the atomic number and the number of _____ are always the same. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … This means that the total number of protons and neutrons in an atom of nitrogen is 14... They are opaque, very hard, and chemically inert, melting only at very high temperatures (generally over 2500 °c).
